Methane Chemistry
Methane, with the chemical composition CH4, is the simplest alkane, or paraffin hydrocarbon, and considered the most potent of the greenhouse gases.
An alkane is any hydrocarbon (a compound containing only carbon and hydrogen) with a general chemical formula of CnH2n+2, C being a carbon atom, H a hydrogen atom, and n an integer. Chemically, methane is also a Group-14 hydride, which means that its a compound made up of hydrogen and atoms from Group 14 of the Periodic Table of Elements. In addition to carbon, Group 14 elements include: silicon, germanium, tin, lead and flerovium.
Greenhouse gases (GHG’s) store heat in the earth’s atmosphere; and in the case of methane, when it oxidizes, it converts into CO2 and other chemicals (CO2 being the most prevalent of GHG’s. The EPA estimates that methane accounts for 10% of all U.S. GHG emissions, but has over 25 times the climate impact of CO2.
Human-Linked Sources of Methane

Methane in the atmosphere has many sources, some of which are biogenic, or naturally occurring, while most have anthropogenic, or human-caused, origins.
Even knowing this, the latest studies indicate that emissions inventories, or calculations made by nations as a way to quantify their climate change responsibility, ought to include biogenic methane emissions sources and sinks (sources of methane absorption and conversion) in their calculations. (Most do not yet.) The greatest natural methane sources include: animal, including human, digestion, animal waste, decomposition, microbial anaerobic oxidation caused by methanotrophs, melting permafrost, and tree and plant respiration.
Quantifying human activity-caused methane pollution is difficult because many sources overlap natural and “unnatural”/human-induced categories. Among these include: agriculture, decomposition at landfills, wildfires/controlled fires, and Net Zero-related tree planting programs. (Yes, even our Carbon Neutral initiatives have complex environmental effects.)
With this said, some mostly-anthropogenic air pollution sources are glaringly obvious: oil and gas mining activities, transportation, and energy production top the list. Oil, gas, and energy are likely the main targets of recent U.S. government initiatives to reduce methane for this reason, among others. Mass emission rates are more easily calculated, leaks are more easily identified and repaired, and large segments of these sectors remain unregulated or under-regulated. This is primarily true of older sites, which are typically not subject to new regulatory standards.
Atmospheric Methane Cycle
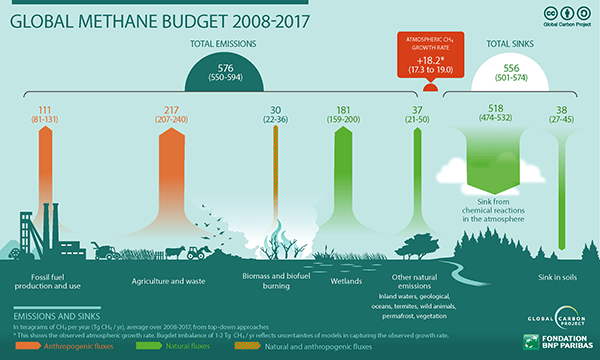
Methane enters and exits the atmosphere through complex networks of emissions sources and sinks. Each individual source and sink cycle is complicated enough on its own, but putting them all together to paint an accurate picture of global methane movement is even more complicated still.
When it comes to the earth’s natural mechanisms for removing methane directly from the air, none is more important than the work of hydroxyl radicals present in the earth’s lower atmosphere (the troposphere and stratosphere).
Hydroxyl radicals (with the chemical formula OH) are formed in the atmosphere through photochemical (light reactions) between water and plant-secreted terpenes. These radicals “steal” elements from neighboring compounds, the two most common chemical reactions of which produce water vapor and CO2 (CO2 itself being the world’s most pervasive greenhouse gas). The breaking down, or oxidation, of methane in the troposphere and stratosphere likely account for approximately 90% of all methane removal from the air.
Climate Impact
Each global warming gas compound has been assigned an impact factor relative to CO2, the world’s most abundant greenhouse gas. Methane’s Global Warming Potential (GWP) is estimated to be between 28-36, meaning that its impact over a 100-year period is, on average, 32 times that of CO2. This is due, in part, to the fact that methane releases CO2 when it is broken down chemically. On average, it is believed that CH4 emitted today lasts about a decade in the atmosphere, while CO2, including what is created in the wake of methane’s chemical breakdown, or oxidation, persists for hundreds of years.
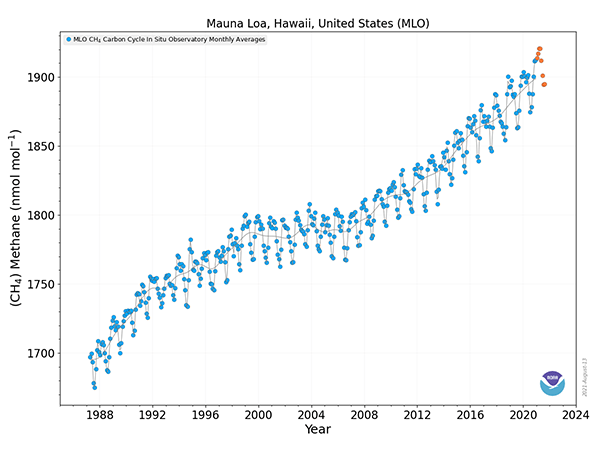
Methane may not last in the atmosphere as long as CO2, but what CH4 ultimately becomes (CO2, O3, and other compounds) will have a longer lasting impact. Unfortunately, methane concentrations in the atmosphere continue to increase every year, adding to changes in our atmosphere.
But methane isn’t all bad. Greenhouse gases in the atmosphere, at least a percentage of them, keep our environment at life-sustaining temperatures. Without some amount of methane present to absorb and radiate energy from the sun’s light, the world would freeze.
Further Reading
- Methane facts on Wikipedia
- Methane facts on Britannica
- Methane and the Gastrointestinal Tract
- Yale e360: Scientists Zero in on Trees
- Emission of Methane from Plants
- Hydroxyl Radical Wiki
- Biochemical aspects of atmospheric methane
- Environmental Change Institute’s Climate Science of Methane
- Interactive Visualization of Global Methane Budgets
- UCAR Center for Science Education—Biochemical Cycles
- National Geographic: Trees Release Flammable Methane (Article)
- EPA: Inventory of US Greenhouse Gas Emissions and Sinks